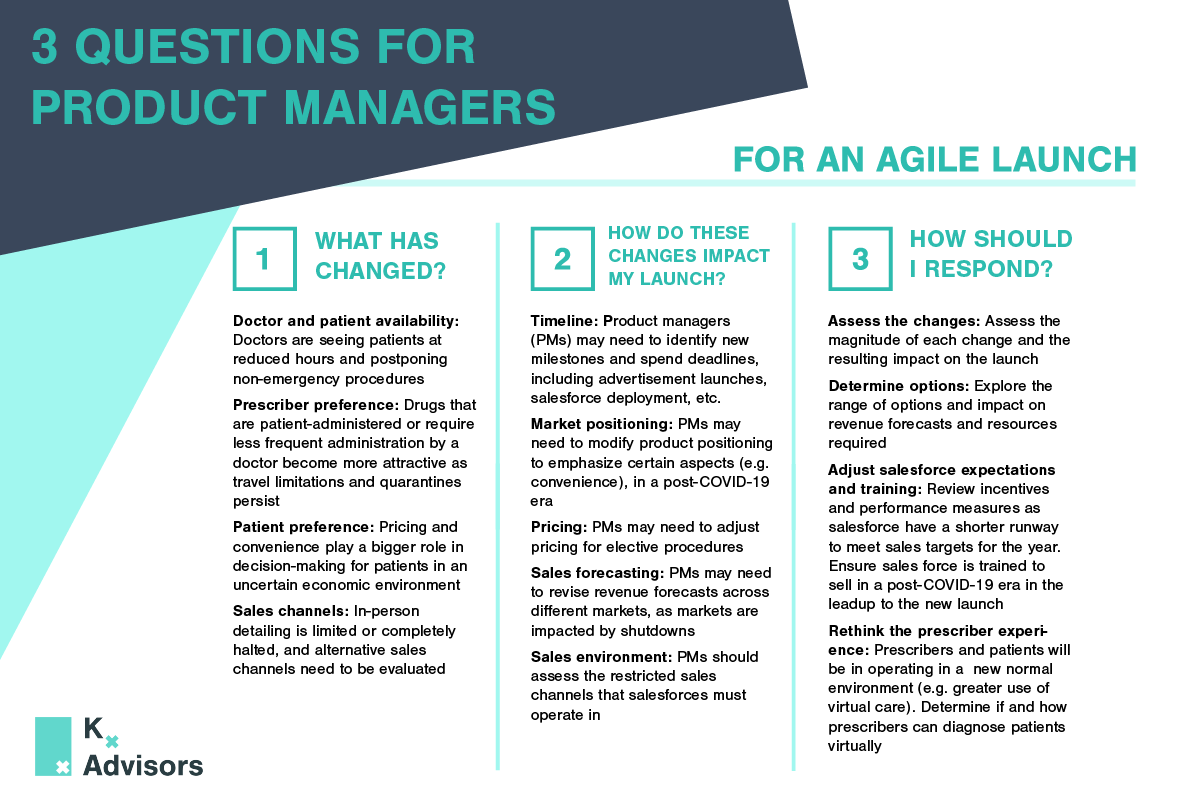
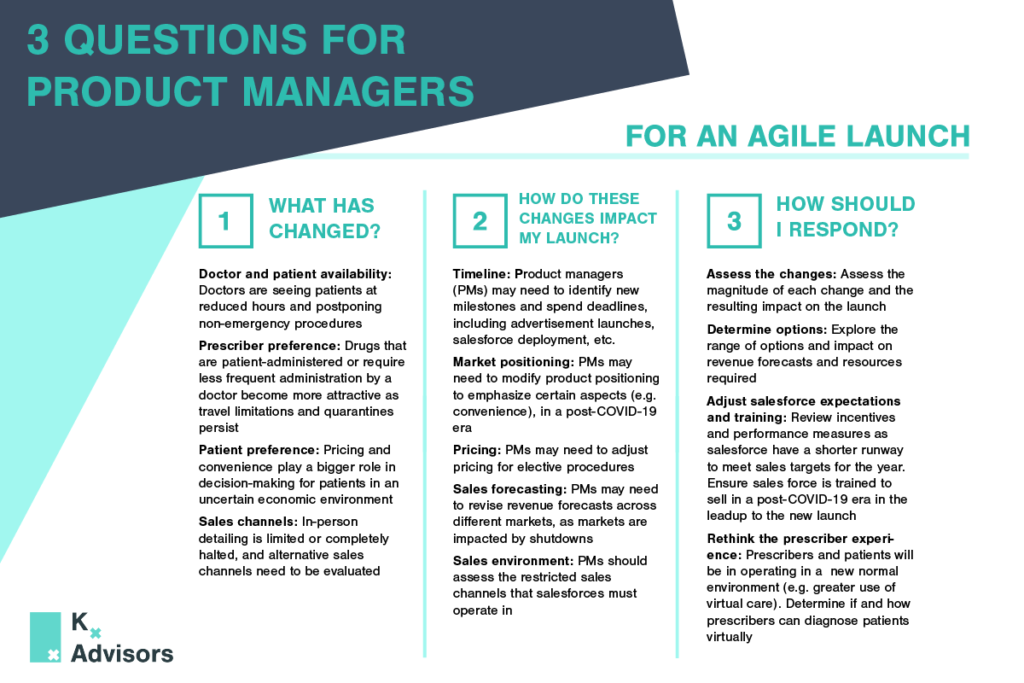
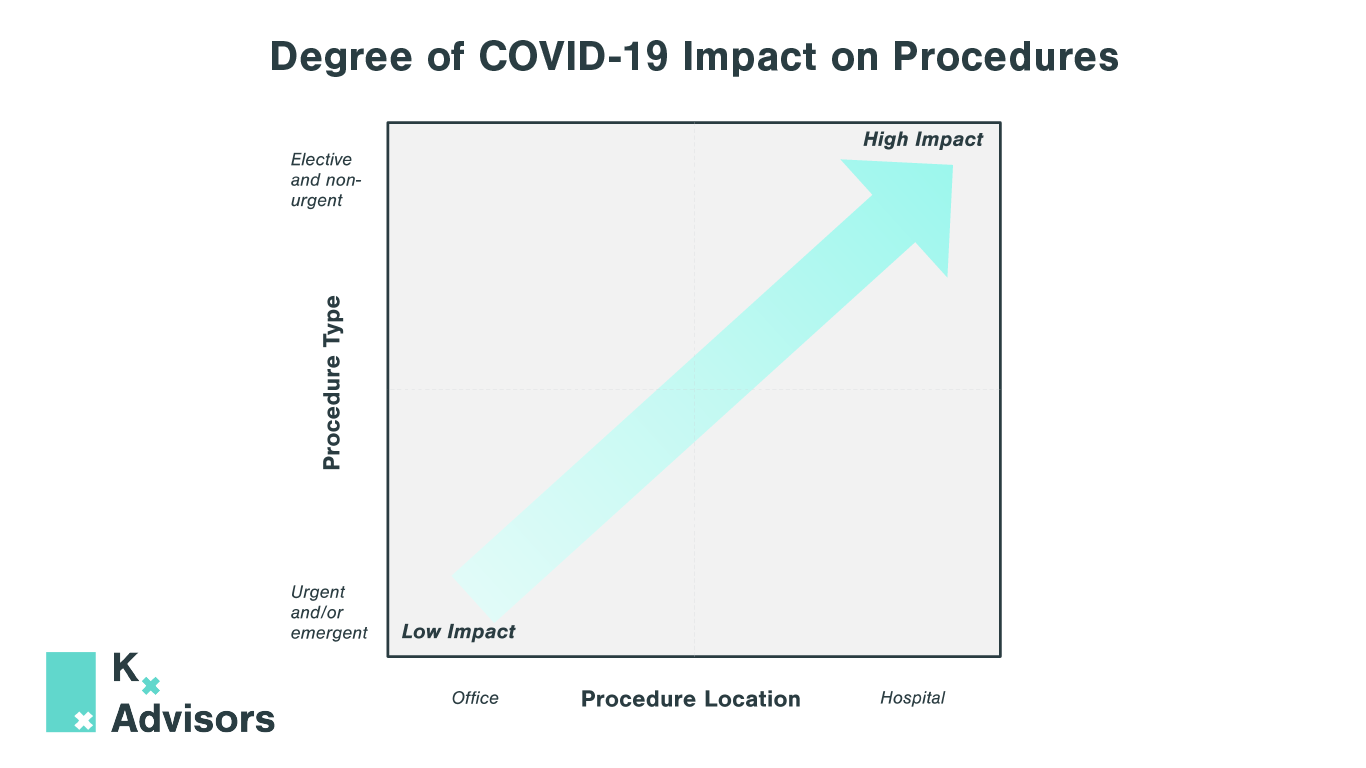
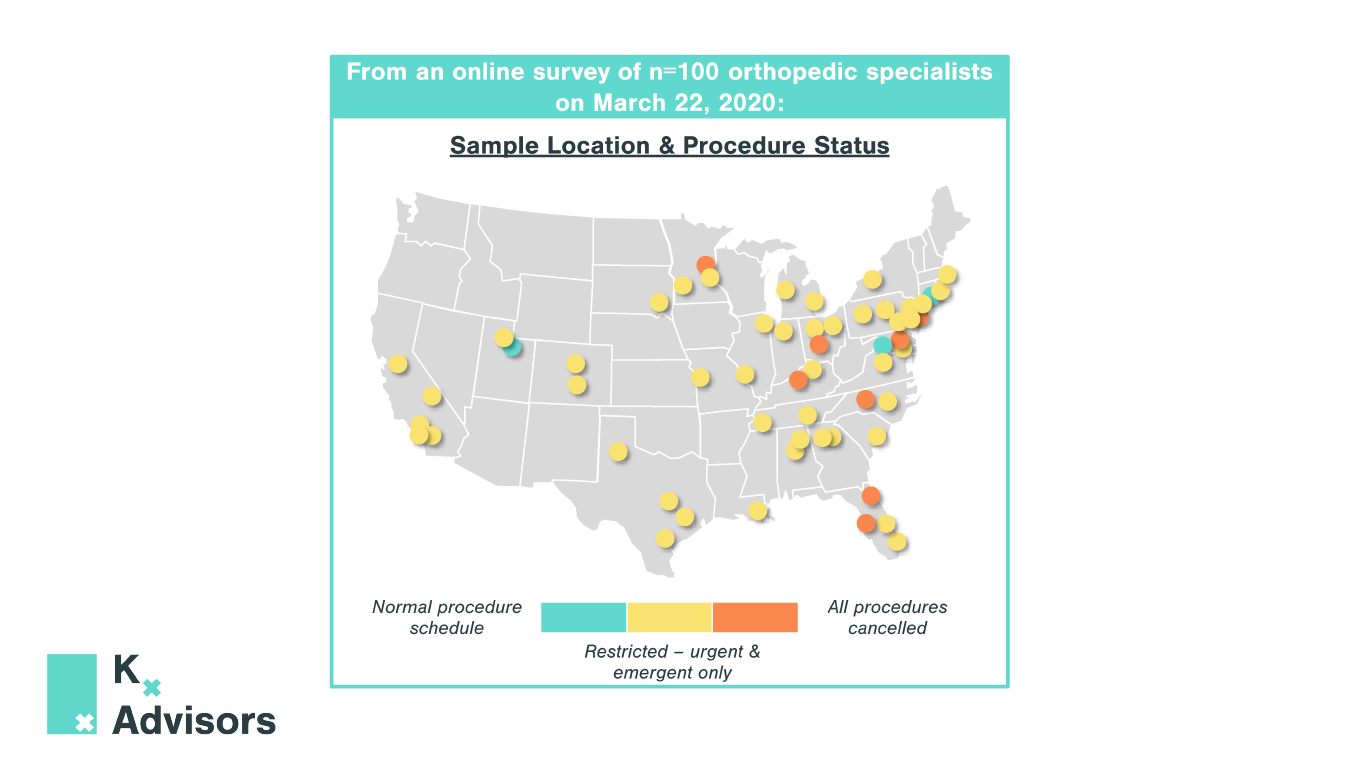
How Medical Practices Can Keep Patients Safe: COVID-19 Best Strategies
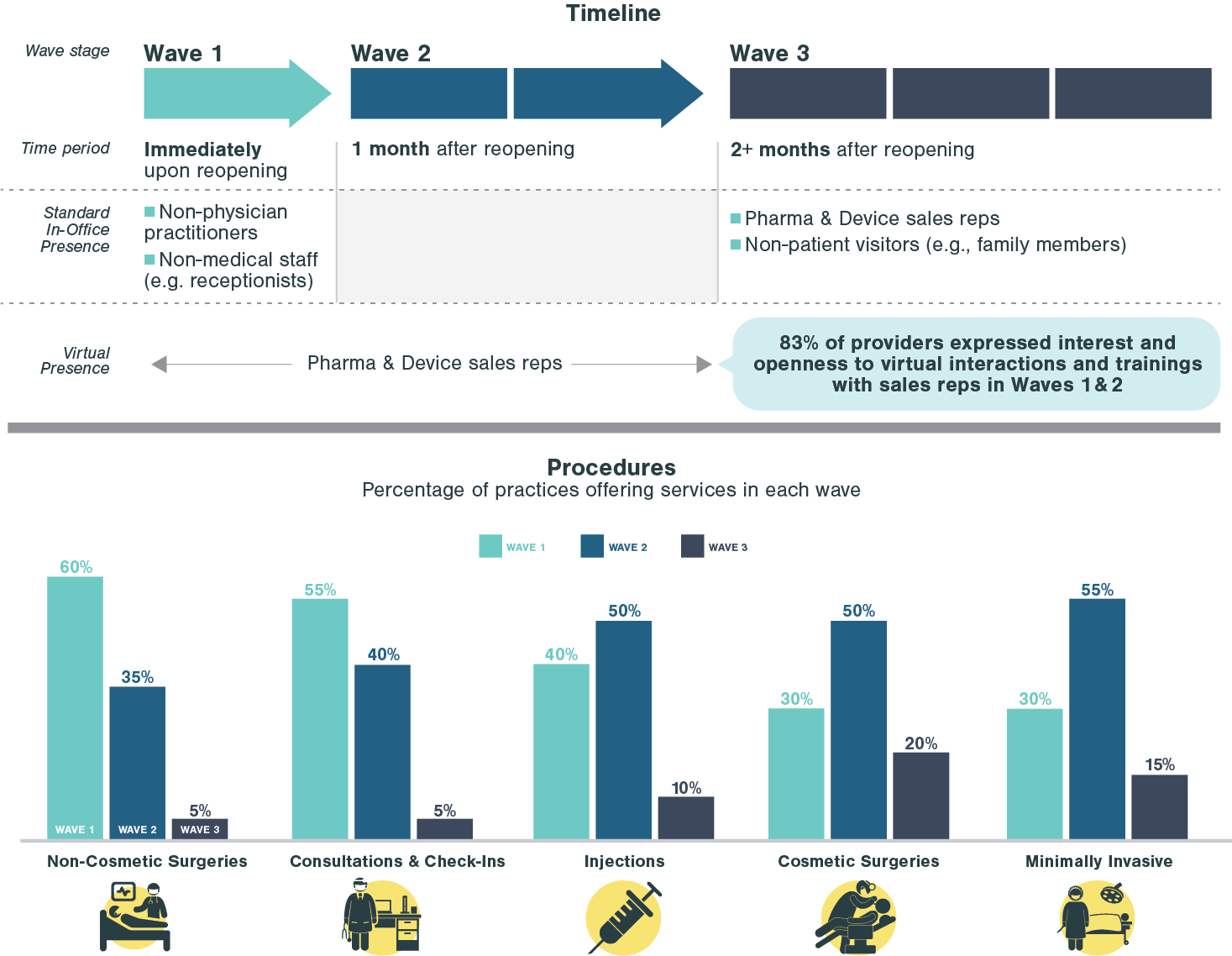
As medical practices reopen to pent-up demand, they must adjust the patient experience to protect both patients and staff from COVID-19. Kx Advisors surveyed one hundred aesthetic physicians across the US to understand the obstacles they face in each step of the patient experience, analyzing the extent of COVID-19’s impact. Building on guidance from the CDC, our experts compiled best practices from these physicians on how they are addressing these pain points. These COVID-19 best strategies aren’t exclusive to aesthetic practices; many can be applied to other clinics and provider practice settings.
COVID-19 Best Strategies For Medical Practices
Applying These Prevention Methods
Our experts compiled these strategies into four main prevention methods that practices can use:
- Reduced time: Practices can limit the time patients spend in the practice, reducing the risk of COVID-19 spread
- Reduced carrier density: Practices can space out appointments, space out waiting room chairs, and eliminate physical check-out to reduce density in the practice
- Transmission prevention equipment: With PPE for both physicians and patients, medical practices can keep staff and patients safe
- Adapted infrastructure: Using an online scheduling program, installing plexiglass at check-in, asking patients to wait in their cars, and leveraging telemedicine can reduce additional risk
Practices also shared suggestions to industry partners on how companies can support them during this reopening period. Top items included product pricing adjustments, expired product replacement, marketing support, limited rep office visits, and help with product-specific guidelines for safe administration. Companies that serve medical practices can adjust their strategy to align with the above guidance and better support their clients.
How Kx Can Help
Kx Advisors is continuing to evaluate business models, deliver top-notch expertise, and make profitable recommendations to our healthcare clients. Our team of experts can help your organization assess how you can best support your aesthetic clients and adapt your corporate strategy to position you for long-term success.