The Role of Stakeholder Education in Rare Diseases
Despite the growing pipeline of orphan drugs, ~95% of rare diseases do not yet have an FDA-approved treatment. This increasing momentum in rare disease R&D has further highlighted the ever-present need for stakeholder education and engagement, which is critical to begin well before drug commercialization. Patients, key opinion leader (KOL) physicians, and other physician specialists supporting treatment are primary players in the rare disease space that influence treatment selection and access to novel therapies. Rare disease stakeholder education plays a critical role at every stage in drug development and commercialization and should be top of mind for rare disease companies regardless of whether they are designing clinical trials or preparing for product launch.
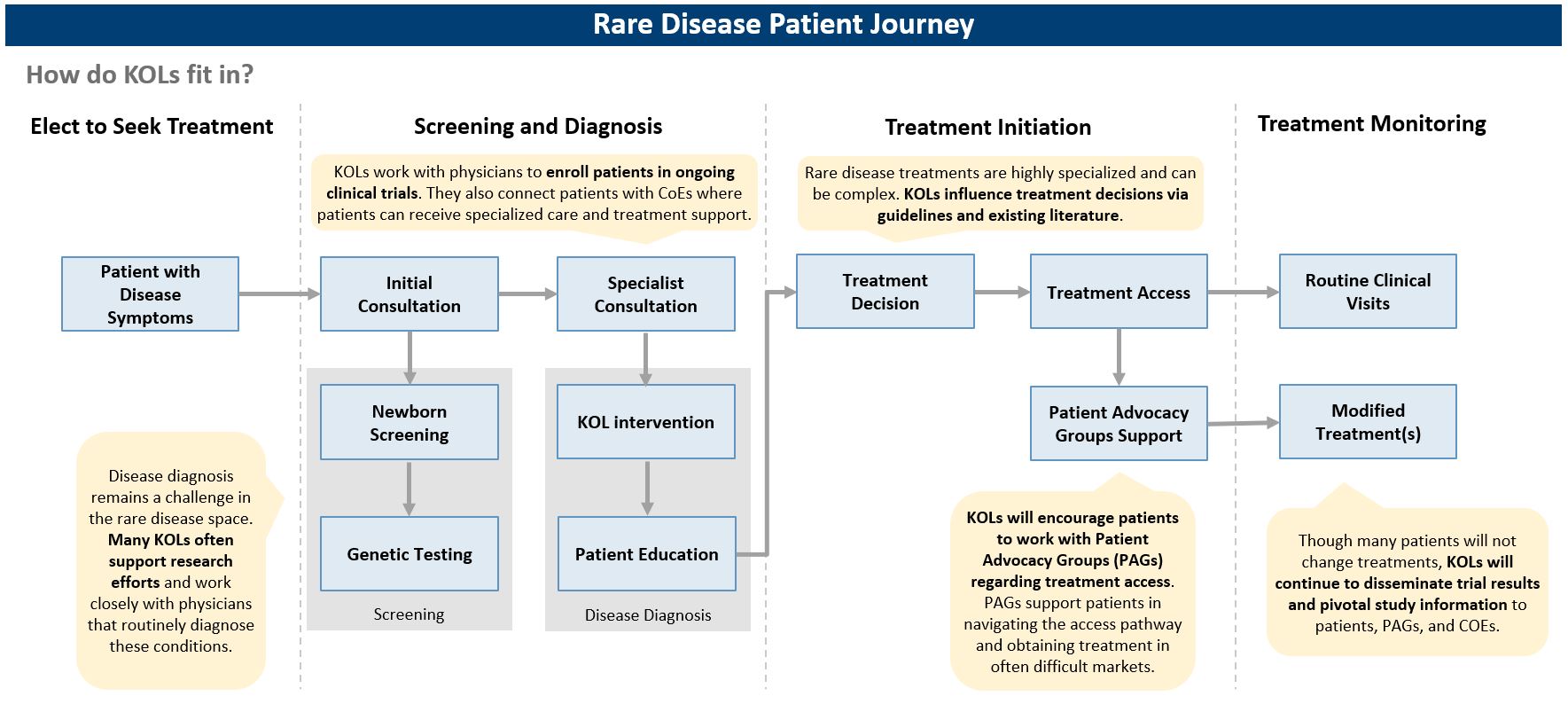
Caption: KOLs play an important role in the patient journey. From diagnosis to treatment access, KOLs support rare disease patients in making important decisions about managing their condition and obtaining the best possible care.
How to Implement Rare Disease Stakeholder Education
Rare disease companies need to engage with different stakeholders to address various pain points along the patient journey and maximize the opportunity for their orphan drug. KOL communities will be especially valuable throughout the drug development process. As a part of tight-knit medical communities, KOLs collectively influence treatment standards and typically have a deep understanding of how access to care and treatment varies across global markets. KOLs will be play important roles in:
- Diagnosis: Timely diagnosis remains a major challenge for rare diseases; on average, patients wait close to 5 years and visit over 7 physicians before an accurate diagnosis is made. Delayed diagnosis can further complicate the patient pathway, often increasing the number of interventions required after the disease has progressed. Dedicated education efforts targeting physicians and patients, as well as improved testing access, can ensure accurate and earlier diagnoses. Companies should look at this as an opportunity to educate stakeholders and develop genetic tests to increase diagnosis rates and maximize patient adoption. While there are currently no genetic tests available for many rare conditions, competition to develop effective diagnostic tests will increase after a disease modifying treatment is introduced to the market. KOLs can also support development of innovative screening techniques, including using artificial intelligence or predictive analytics with electronic health record data to identify patients at high risk of an undiagnosed rare disease.
- Drug development: KOLs play an integral role in drug development, ranging from supporting animal model design, clinical trial design, and designing clinical endpoints (e.g., novel disease severity / quality of life scales). As disease experts, KOLs understand needs, market access challenges, and adoption drivers and should be considered an asset to pre-commercialization efforts for rare disease companies. KOL in-depth expertise and knowledge should be leveraged for refining target product profiles (in particular via advisory boards), understanding treatment barriers across markets, estimating the size of local patient populations, selecting sites and recruiting patients for clinical trials, and supporting market access and reimbursement decisions for regulatory bodies and payers. Companies will greatly benefit from early and global KOL engagement throughout the drug development process – whether as consultants or scientific investigators.
- Launch planning: KOLs are very familiar with the gaps in the existing patient pathway, and, post drug launch, can provide insights on ideal future-state diagnosis and referral patient pathways. Rare disease companies should continue to collaborate with KOLs to streamline the access pathway and demonstrate their long-term commitment to a given patient population. Post-launch trials also offer an opportunity for KOLs to disseminate information and develop stakeholder education deliverables about achieved health outcomes and / or pivotal trial results with physicians and patient advocacy groups (PAGs). Over time, KOLs become strong advocates for drugs, especially those that they have supported through development, and can be important allies as companies move towards commercialization.
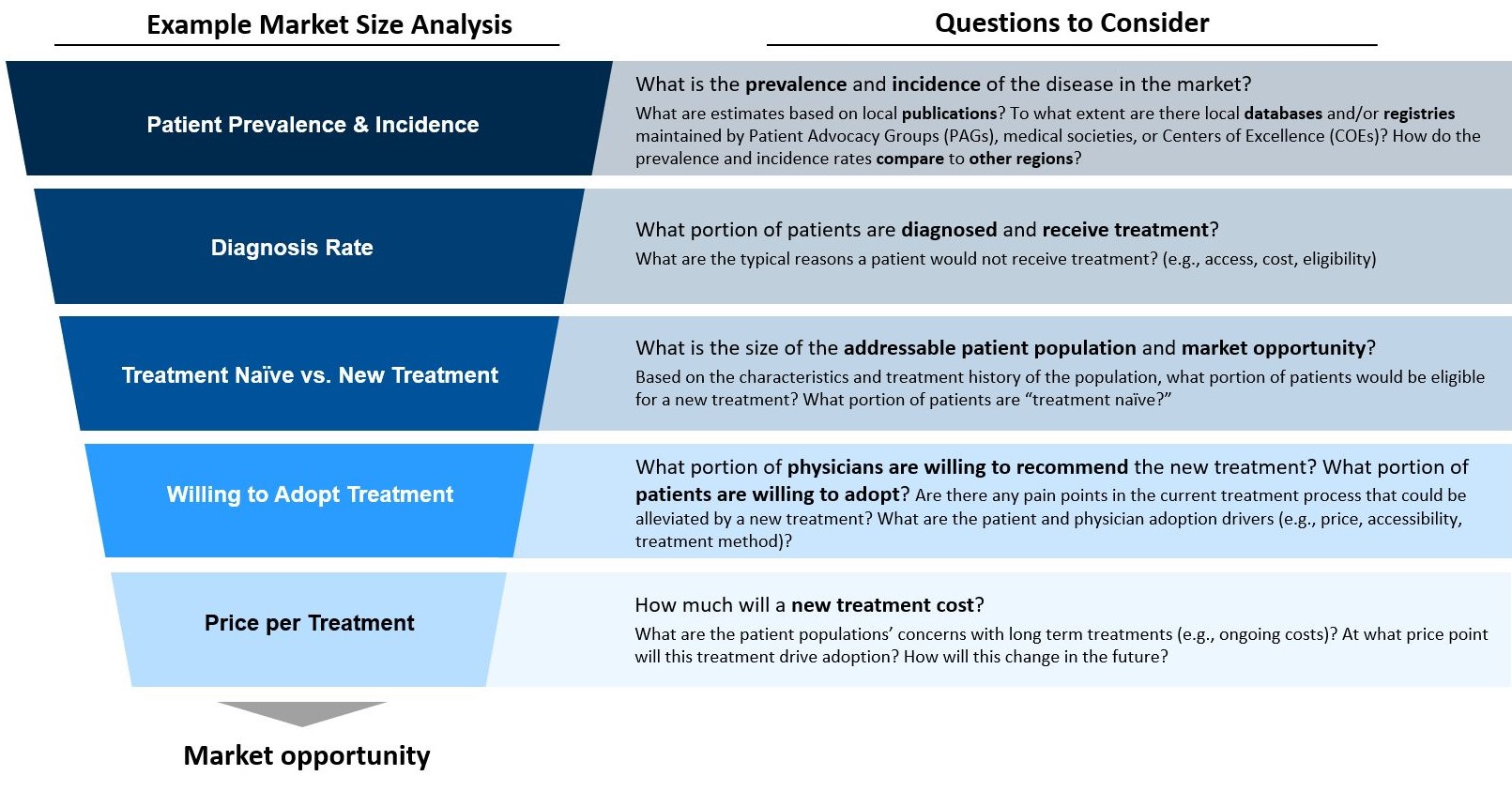
Caption: Companies looking to launch a new drug should focus on how they can work with KOLs and other stakeholders to best understand local market opportunities, including disease prevalence/incidence, frequency of diagnosis, and likelihood of treatment adoption.
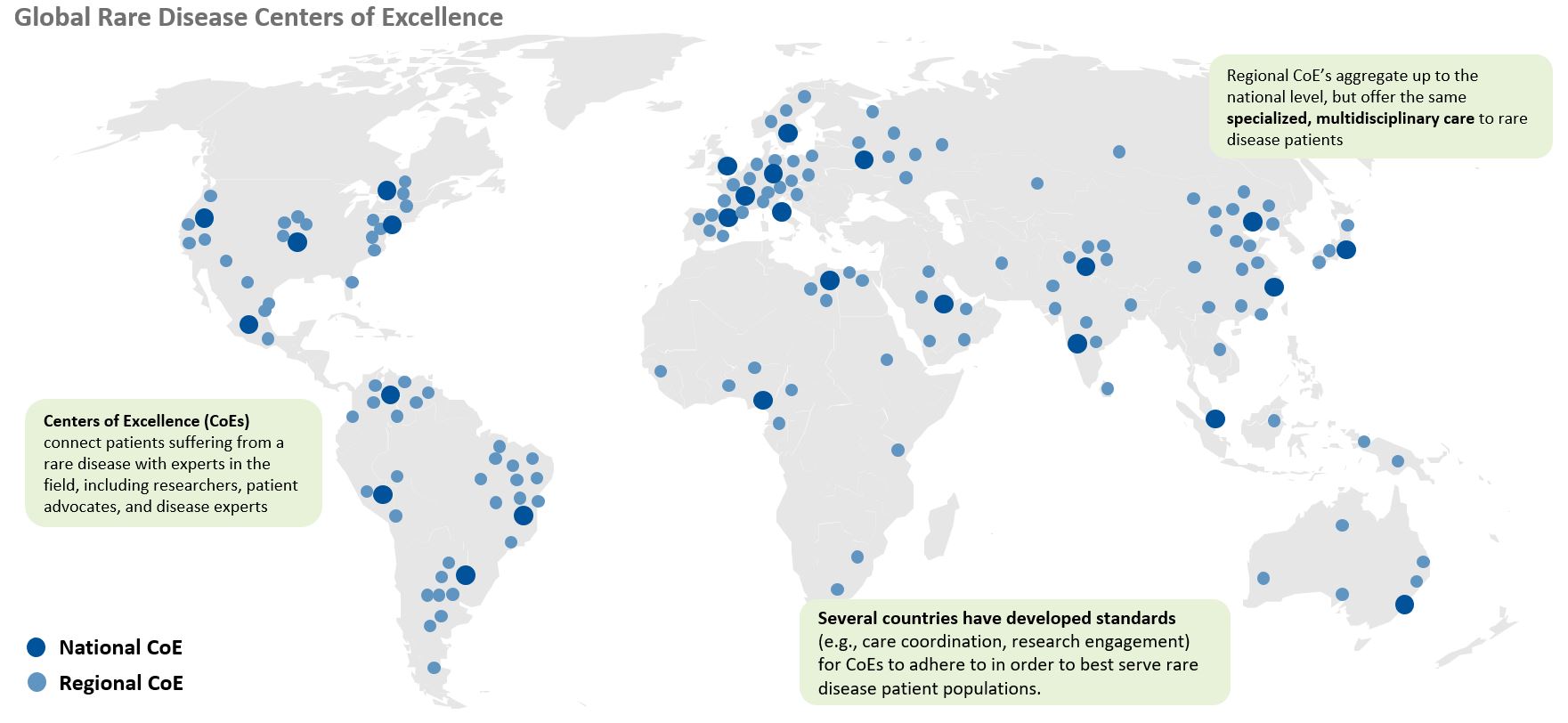
Centers of Excellence (COEs) are another critical component of rare disease stakeholder education. COEs, in conjunction with KOLs and their reference networks, offer rare disease patients specialized care and expertise. COEs combine clinical research, knowledge, and treatment services to become a regional or global “hub” for undiagnosed or recently diagnosed patients. The multidisciplinary approach they take is not only valuable to patients, but also biopharma companies as they enter commercialization stages. COEs are a reliable source of support for companies, referring patients from their centers and supporting commercial activities later down the road. A strong relationship with COEs and their KOLs can mean success for rare disease companies looking to work closely with patient populations and demonstrate long term commitment.
Caption: Centers of Excellence are a critical component of the rare disease patient pathway. Patients seek diagnosis support, treatment advice, and care coordination from experts at COEs across the globe.
KOLs and COEs are key stakeholders in the rare disease space and should be top of mind for companies looking to enter the space. As competition within the market increases, how companies work with KOLs to leverage their expertise and best understand the treatment landscape will become a key differentiator. KOLs, though critical to the market, are not the only stakeholders that companies should keep an eye on. In the next part of these series, we will share why patient advocacy groups are an important ally for both patients and rare disease companies, and how they can successfully work together to introduce new treatments to the market.